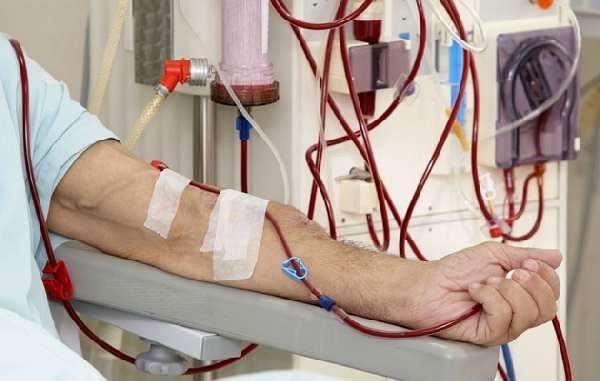
FDA permits marketing of first catheter-based systems used to create vascular access for hemodialysis patients
The U.S. Food and Drug Administration has permitted marketing of two catheter-based devices designed to create an arteriovenous (AV) fistula in patients with chronic kidney disease in need of hemodialysis. The devices are designed […]