FDA declines proposed Rituxan, Herceptin biosimilars from Celltrion
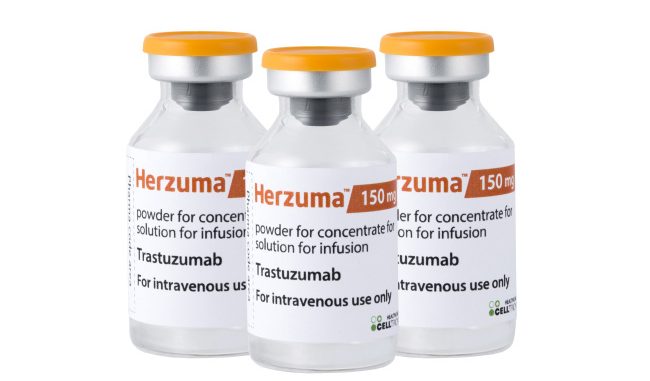
The US Food and Drug Administration has rejected Celltrion’s submissions for biosimilars to Roche drugs Rituxan and Herceptin. According to media reports, the company received complete response letters for Herzuma (trastuzumab; CT-P6) and Truxima (rituximab; […]